CGHS and CS(MA) Rules: Revised ceiling rates for reimbursement of the cost of Cardiac pacemaker, AICD, Combo-device, Rotablator and Aortic Stent Graft
Government of India
Ministry of Health and Family Welfare
Department of Health & Family Welfare
Directorate General of CGHS
Office of the Director, CGHS
No: S-11011/29/2018-CGHS(HEC)/ DIR/CGHS
Nirman Bhawan, New Delhi
Dated the 6th August, 2018
OFFICE MEMORANDUM
Subject:- Revision of ceiling rates for reimbursement of the cost of Cardiac pacemaker, AlCD, Combo-device, Rotablator and Aortic Stent Graft for beneficiaries of CGHS/CS(MA) Rules.
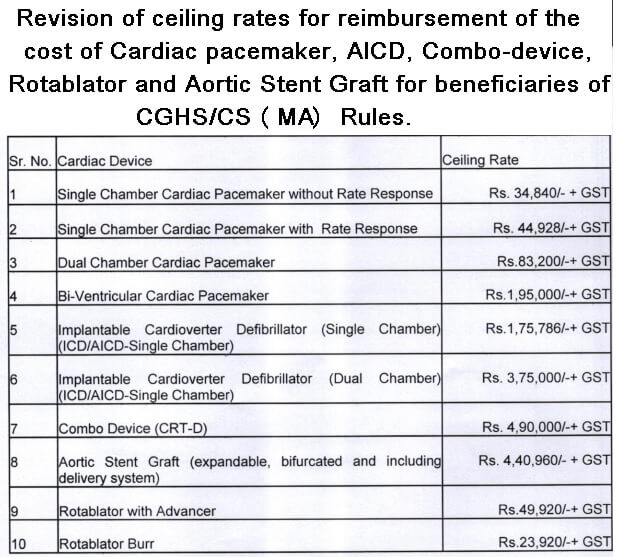
With reference to the above subject attention is drawn to the OM No 12034/02/2014/Misc./- CGHS D.lll dated 22nd July 2014 vide which ceiling rates for reimbursement of the cost of Cardiac pacemaker, AICD, Combo-device, Rotablator and Aortic Stent Graft for beneficiaries of CGHS/CS (MA) Rules were prescribed and to state that the matter has been reviewed by the Ministry and it is decided to revise the ceiling rates as per the details given under:
| Sl.No. |
Cardiac Device |
Ceiling Rate |
|
1
|
Single Chamber Cardiac Pacemaker without Rate Response | Rs.34,840/- + GST |
|
2
|
Single Chamber Cardiac Pacemaker with Rate Response | Rs. 44,9281-+ GST |
|
3
|
Dual Chamber Cardiac Pacemaker | Rs.83,200/-+ GST |
|
4
|
Bi-Ventricular Cardiac Pacemaker | Rs.1,95,000/-+ GST |
|
5
|
Implantable Cardioverter Defibrillator (Single Chamber) (ICD/AICD-Single Chamber) |
Rs.1,75 786/-4+ GST |
|
6
|
Implantable Cardioverter Defibrillator (Dual Chamber) (ICD/AICD-Single Chamber) |
Rs. 3,75,000/-+ GST |
|
7
|
Combo Device (CRT-D) | Rs, 4.90,000/-+ GST |
|
8
|
Aortic Stent Graft (expandable, bifurcated and including delivery system) |
Rs. 4,40.960/- + GST |
|
9
|
Rotablator with Advancer | Rs.49,920/-+ GST |
|
10
|
Rotablator Burr | Rs.23,920/-+ GST |
2. Other terms and conditions prescribed under OM No 12034/02/2014/Misc./-CGHS D.III dated 22nd July 2014 shall remain unchanged.
3. These rates shall remain valid till the rates for the above devices are notified by National Pharmaceutical Pricing Authority (NPPA).
4. Issued with the concurrence of SS&FA, Ministry of Health & Family Welfare vide CD – No.1295 dated 25.07.2018.
(Dr. Atul Prakash)
Director, CGHS
[https://cghs.gov.in/showfile.php?lid=5117]

COMMENTS